„Bone metastases are a significant impairment of life for over 1.2 million patients in the US and the EU each year“*¹
„Bone metastases are a major cause for morbidity, characterized by severe pain, impaired mobility, pathologic fractures, spinal cord compression, bone marrow aplasia and hypercalcemia“*²
“75% of all breast and prostate cancer patients develop bone metastases during the course of their disease“*²
1) Extrapolated based on: Incidence of bone metastases in patients with solid tumors: analysis of oncology electronic medical records in the United States, Hernandez et al. BMC Cancer (2018) 18:44, DOI 10.1186/s12885-017-3922-0
2) Oncology Reviews 2017; 11:321
Englisch
German
Target local activity
Significant reduction of cancer tissue
Stop bone resorption
Reduction of bone metastases
Regain bone density
Less pain
More mobility
Better quality of life after cancer treatment
We are a Development Company
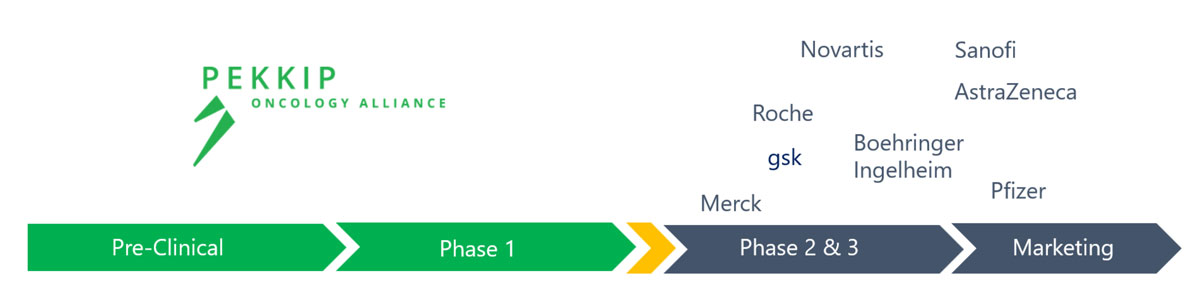
Focusing on early research success, where there is significant promise, we are able to develop the asset while maintaining financial diligence.
We capture the significant value increase after successful Phase 1, with limited resources required.
Larger Pharma-Companies have the right leverage to perform go to market strategies and investment heavy later clinical studies.

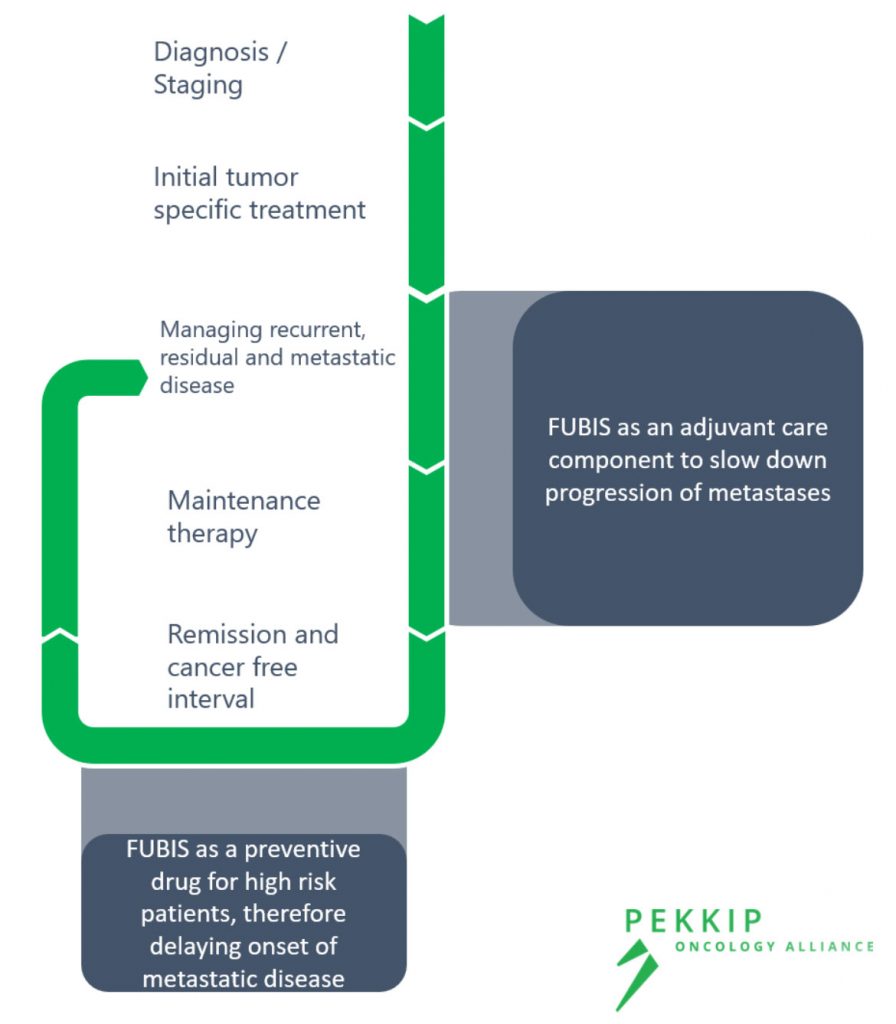
- FUBIS could be used across all types of breast cancer that are present with bone metastases.
- Treating patients in adjuvant setting, in parallel with ongoing breast cancer regimens.
- Treating patients during chemotherapy-free intervals to prolong time-to-relapse and avoid skeletal related events.
- Application via infusion on 28 days intervals.
- Administration through out-/in-patient settings, no need for monitoring.
Our collaboration allows us to work with leading scientists.

Pre-Clinical Research & Imaging
University of Schleswig- Holstein