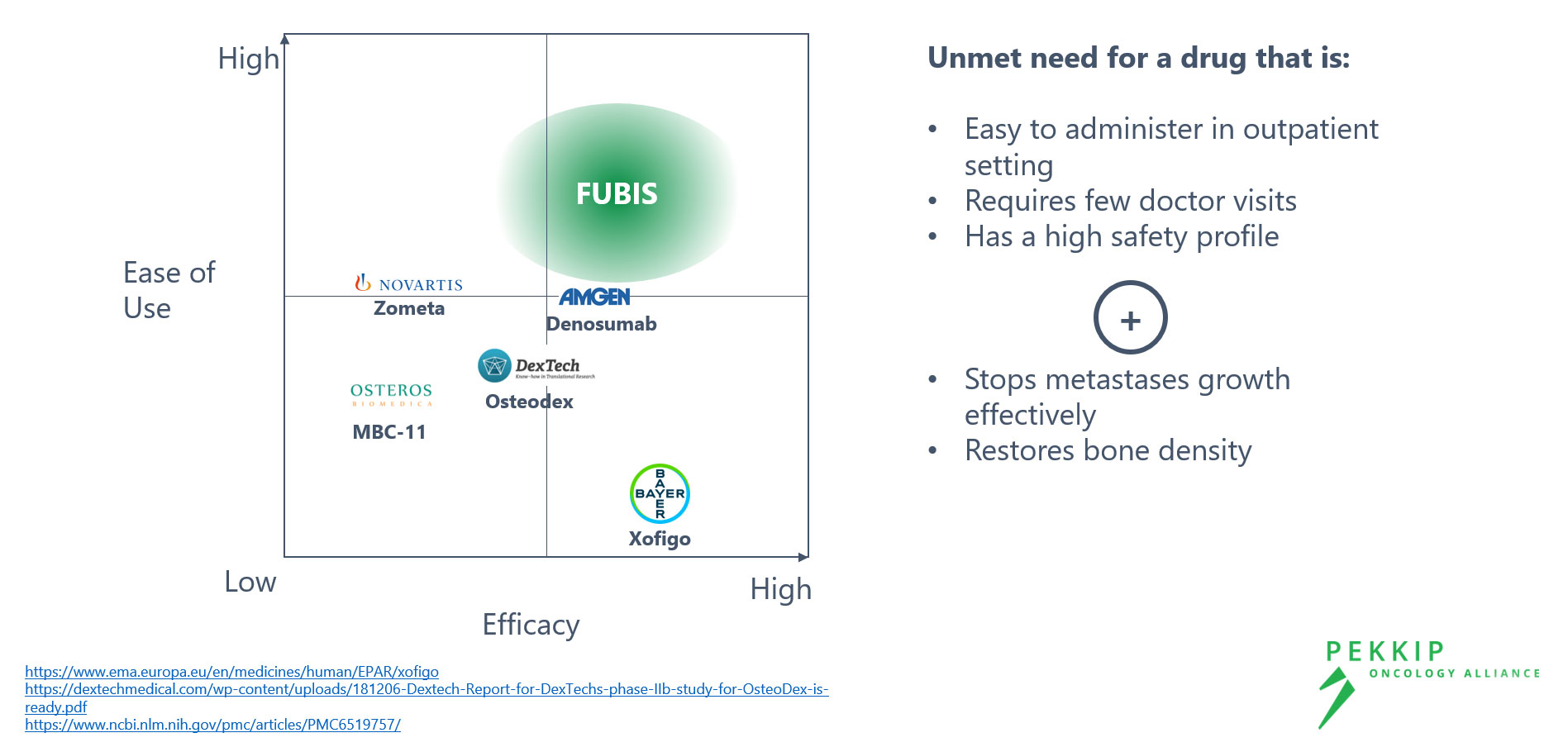
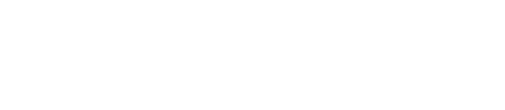FUBIS – Targeted Inhibition of Bone Resorption and Reduction of Tumor Size
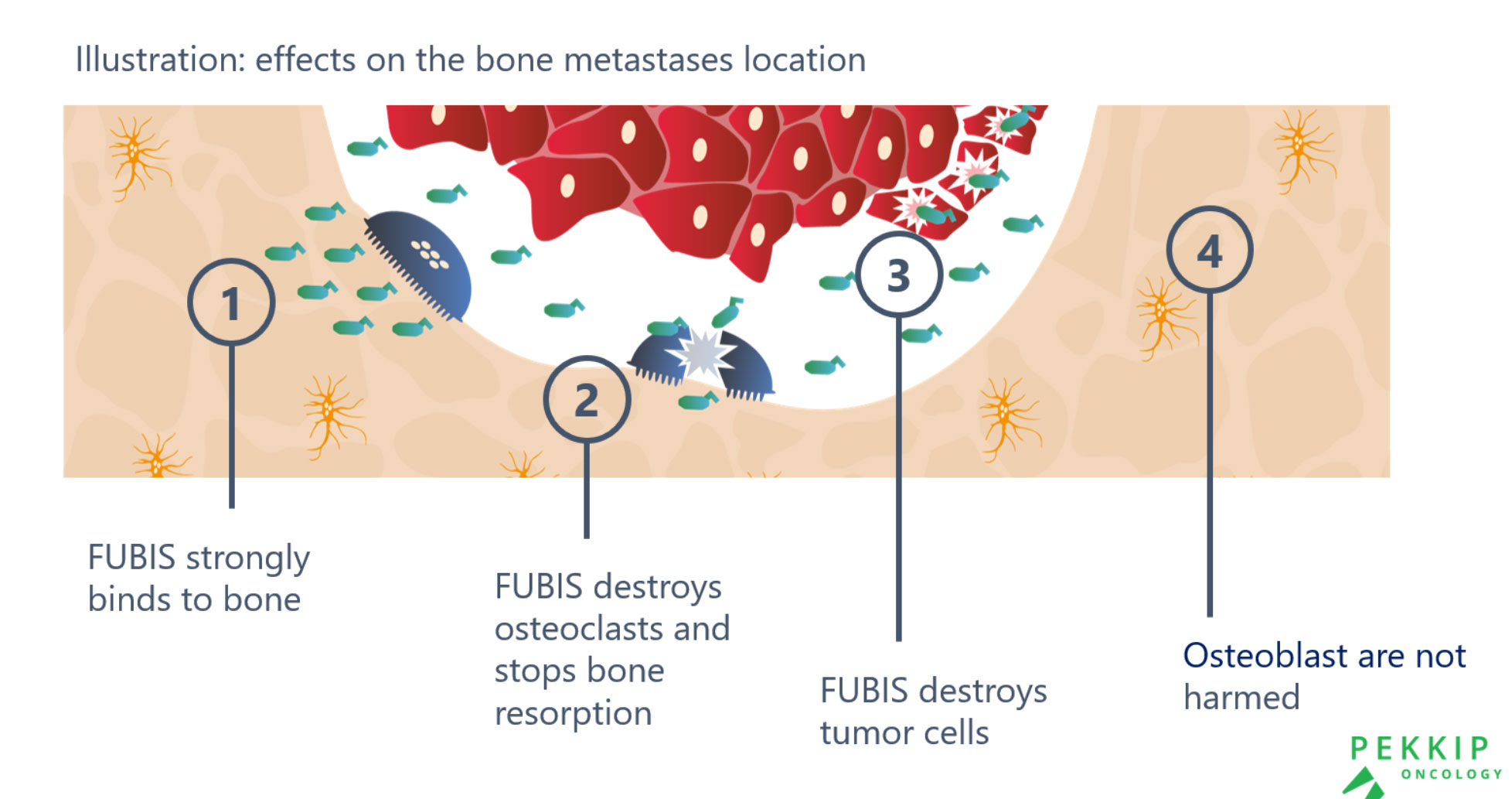
Pre-clinical tests find FUBIS safe, well-tolerated and effective against Bone Metastases
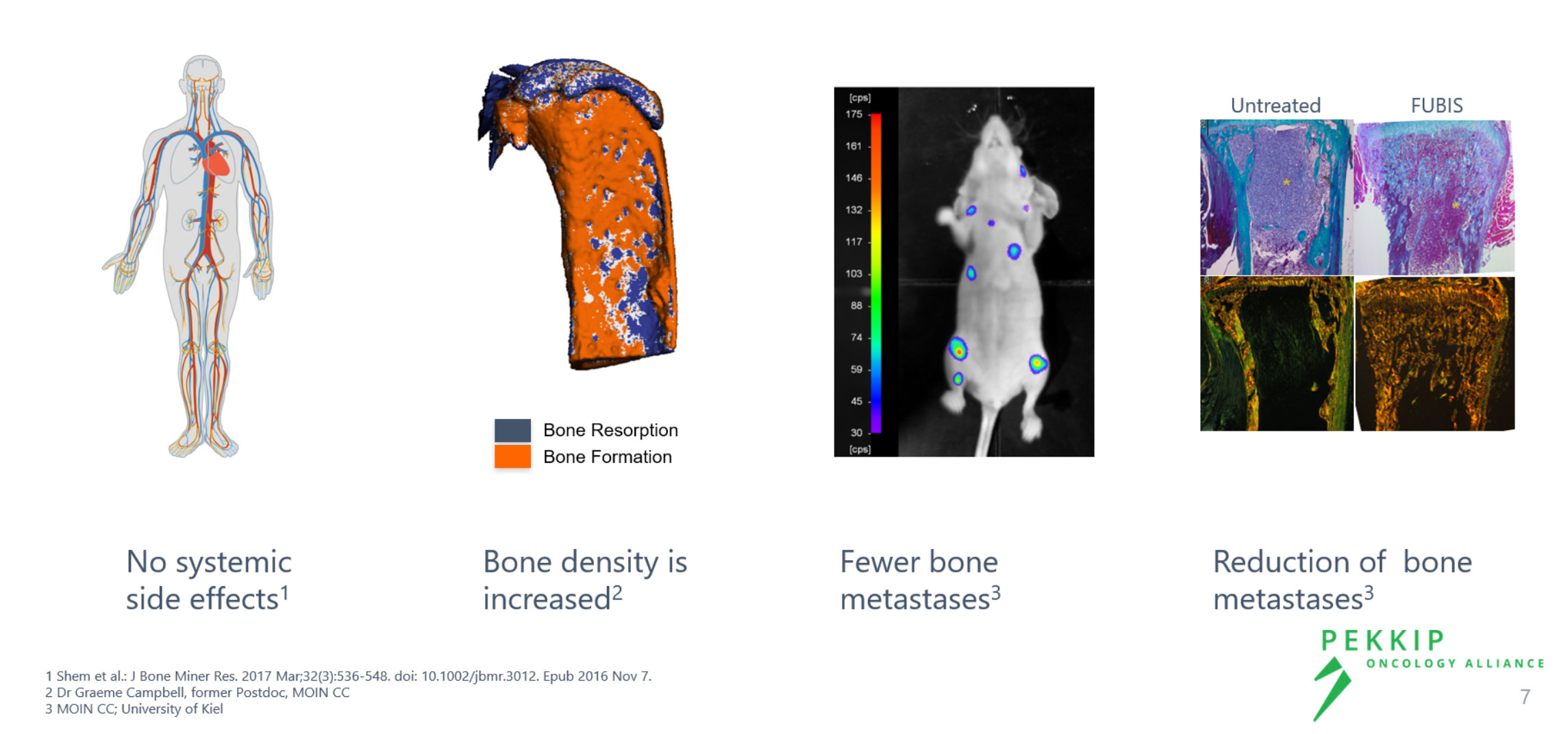
Our Linker Technology is the crucial element to bring together desired characteristics
The competition has not found the right balance between risk profile and efficacy
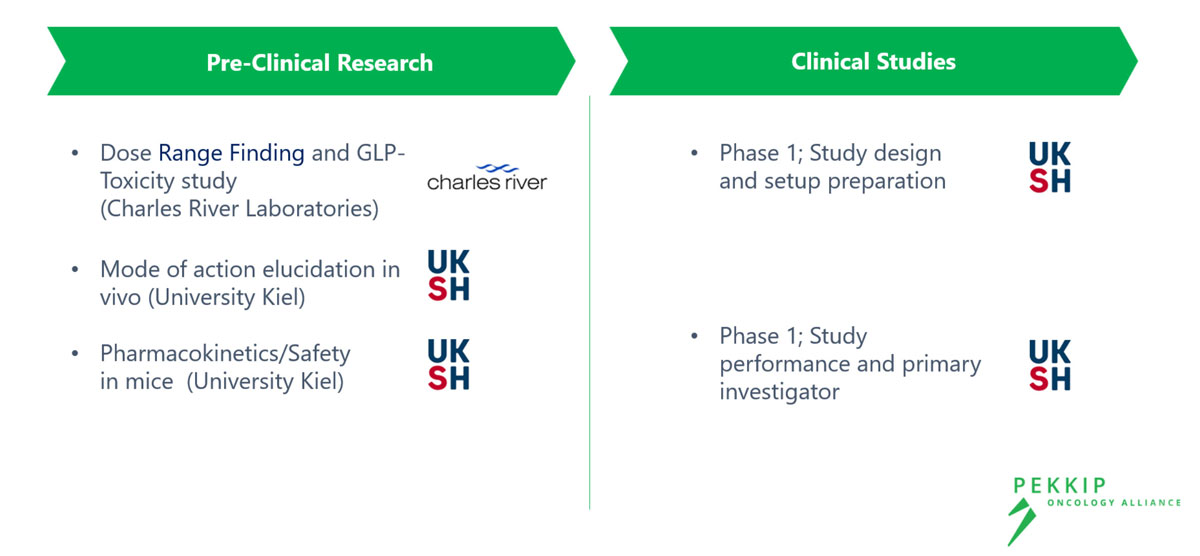
Our Research Activity for FUBIS
Key Metrics for Commercial Scenarios

PEKKIP Oncology Alliance holds an exclusive, worldwide license to the Linker Technology and resulting molecules:
- All of Prof. Dr. Schott‘s rights in patents or patent applications deriving from or being based upon PCT/EP2011/063326
- Prof. Dr. Schott‘s secret technical and scientific knowledge relating to the invention (Contract Know-How)
Prof. Dr. Schott maintains the right to use the invention for non-commercial purposes but any potential publication of results from such activities are reportable to PEKKIP
The patents cover the full product class of bisphosphonates that use the proprietary conjugation-technology which is used within FUBIS
Resulting from this, all chemical structures that link bisphosphonate and other substances based on this structure are covered by the patent – meaning we could also replace Alendronate or 5-FdU by other compounds and stay within the patent protection
In addition the patent and the commercial rights license to Pekkip Oncology Alliance would cover any other substance with the described specifics that might be suitable for commercialization